Discovery of New Solid Catalysts for Water Electrolysis
Green hydrogen — produced from water electrolysis by using sustainable electricity — is getting more attention due to its potential to be used as energy carrier as well as building block for various industrial processes. Among both half-reactions of water electrolysis, Oxygen Evolution Reaction (OER) is kinetically more challenging and it requires advances in the development of innovative electrocatalysts.
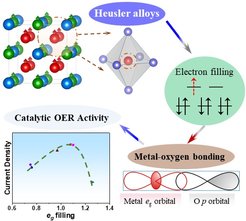
PD Dr. Harun Tüysüz (Max-Planck-Institut für Kohlenforschung), Prof. Dr. Claudia Felser (Max-Planck-Institut für Chemische Physik fester Stoffe) and co-workers have discovered a new type of OER electrocatalyst. A variety of Co2YZ type Heusler compounds with tunable physicochemical properties and well-defined topological surfaces were designed and demonstrated to effectively split water into oxygen and hydrogen. The systematic electrocatalytic investigation of the KOFO team proved a solid correlation between electron filling of d-orbitals of cobalt centers and their OER activities. The materials shoved a volcano-shaped activity curve where the higher catalytic current was obtained for eg orbital filling approaching unity.
This work demonstrates proof of concept implementation of Heusler compounds as a new class of OER electrocatalysts, and the effect of orbital occupation on their catalytic performances. The results of this study were recently accepted for publication in Angewandte Chemie International Edition as “Hot Paper”.
Publication: Yu, M.; Li, G.; Fu, C.; Liu, E.; Manna, K.; Budiyanto, E.; Yang, Q.; Felser, C*, Tüysüz, H.* Tunable eg orbital occupancy in Heusler compounds for oxygen evolution reaction, Angew. Chem. Int. Ed.,2021












