Functional Materials
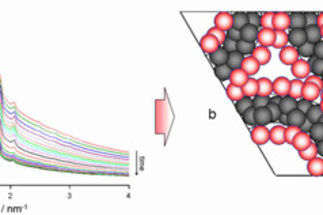
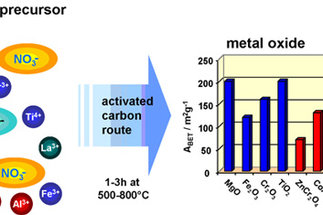
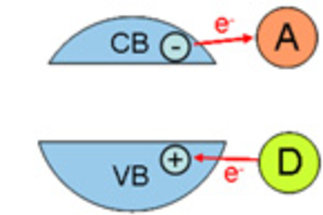
Solid materials with a given functionality are of relevance for many fields in chemical research. Surface functionalities are of specific interest and determine the properties of materials for processes that proceed at the interface of a solid and a fluid. Typical processes are heterogeneously catalyzed reactions, adsorption processes, and electrochemical reactions. Crucial factor for efficient processes are high specific surface areas that can be realized either by generation of pores in a solid or by formation of nanoscopic particles.