A bit of silver boosts the production rate of green hydrogen
outstanding publication in 'Angewandte Chemie' on a highly relevant subject
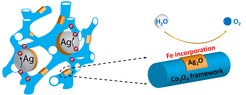
A team of scientists at the Mülheim Chemistry Campus (MPI für Kohlenforschung + MPI for Chemical Energy Conversion) presents the possibility of electrocatalysts based on active cobalt oxide with silver coupling, thus paving the way for more effective production of green hydrogen - the sustainable "oil" of tomorrow.
The implementation of diverse renewable energy technologies is the ultimate solution to the crisis of fossil fuels and climate change in our generation. One of the most promising renewable energy technologies is water electrolysis for the production of green hydrogen, which is called as the clean “oil” of tomorrow. Promoting green hydrogen in the energy system as well as chemical industries is taking place in many countries around the world. This includes Germany: the Federal Government of Germany recently released a new National Hydrogen Strategy and established National Hydrogen Council. “In doing so, we are creating jobs for many young people, strengthening Germany’s leadership on technology, and helping to reach international climate targets” said Dr. Gerd Müller, Federal Minister for Economic Cooperation and Development.
Water electrolysis consists of two half reactions, producing hydrogen and oxygen at cathode and anode, respectively. The anode reaction, namely oxygen evolution reaction (OER), is kinetically more challenging and thus requires much more electrical energy comparing the other half reaction, hydrogen evolution reaction. By bar, most of benchmarking OER catalysts are based on noble metals, such as iridium and ruthenium oxides. The scarcity and high cost of noble metals hinder their practical application, especially in a scale that deals with global supply of renewable energy. An alternative solution is to design OER catalysts using earth abundant metals without sacrificing the catalytic activity.
Mingquan Yu, PhD student in the research group of Priv.-Doz. Dr. Harun Tüysüz at the MPI für Kohlenforschung and Rebeca Gomez Castillo, PhD student in the Department of Inorganic Spectroscopy of Prof. Serena DeBeer at the MPI CEC, are both students of the IMPRS-RECHARGE. Together with other scientists from both Max Planck Institutes in Mülheim, such as Priv.-Doz. Dr. Clauda Weidenthaler, they have published a study within the Collaborative Research Centre/TRR 247 in the journal 'Angewandte Chemie' as "VIP" (Very Important Paper).
Members of the Tüysüz research group have been focusing on design and development of cobalt-based oxides as promising OER catalysts to speed up the hydrogen production. Scientists of both institutes demonstrated the beneficial role of silver on the OER activity of cobalt oxides. Silver was found to be present in two phases playing dual role on cobalt catalyst: (1) metallic silver nanoparticles embedded in ordered mesoporous cobalt oxide increase the conductivity of electrocatalyst, and (2) ultrasmall silver oxide clusters (~ 2 nm) on the surface cause the electrochemical activation via uptake of iron impurities from KOH electrolyte. Due to the synergy of these two silver species, adding a bit of silver (less than 4 at. %) brings a more than twofold enhancement of the reaction rate, by delivering a catalytic current density of 211 mA/cm2 at 1.7 V, in comparison to pristine cobalt oxide (102 mA/cm2). This study not only puts forward a direct way to construct active cobalt oxide based electrocatalysts with silver coupling for green hydrogen generation, but also provides experimental evidences on the active role of iron species originated from KOH electrolyte.
Original Publication: Yu, M., Moon, G., Castillo, R., Weidenthaler, C., DeBeer, S., Tüysüz, H*. Dual Role of Silver Moieties Coupled with Ordered Mesoporous Cobalt Oxide towards Electrocatalytic Oxygen Evolution Reaction. Angewandte Chemie International Edition. doi.org/10.1002/anie.202003801.












