New on-DNA chemistry for efficient drug discovery
Scientists from the Max-Planck-Institut für Kohlenforschung, the RWTH Aachen and the University of Bielefeld published their latest findings in nature chemistry
A research team lead by scientists from the Max-Planck-Institut für Kohlenforschung has introduced a new functionalization strategy in the development of DNA-encoded libraries (DELs) — a key technology in pharmaceutical research. Their new study, now published open access in nature chemistry, introduces a method that broadens the range of chemical modifications possible on DNA-tagged molecules.
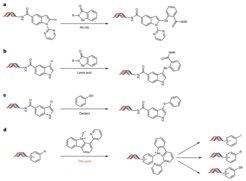
DELs allow pharmaceutical companies to screen billions of small molecules in parallel to find promising drug candidates. Each molecule carries a unique DNA barcode, enabling fast identification of potential hits after binding to a biological target. This makes the approach highly efficient and cost-effective. However, because the compounds are attached to sensitive DNA strands, the chemistry used in DELs must be mild. This limits the structural diversity of such libraries.
The team developed a strategy to carry out C–H functionalization directly on DNA-linked molecules. The process allows chemists to modify otherwise inert parts of a compound, even after DNA attachment. This opens the door to late-stage diversification without damaging the DNA. The reaction relies on specially designed selenoxide reagents that work under DNA-friendly, aqueous conditions and install a versatile chemical handle for further transformations. The approach is compatible with many common structures in medicinal chemistry, including indoles, anilines and piperazines. By enabling access to new regions of chemical space, the method expands the design possibilities for future DELs and helps bring more diverse compounds into screening pipelines.












