Catalytic synthesis of alpha olefins from carboxylic acids: the Ritter group provides a blueprint for this area in ‘Nature Chemistry’
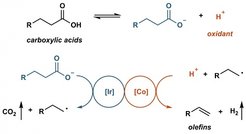
Fatty acids have been thought as the sustainable alternative of petroleum feedstocks for alkene synthesis. However, all efforts in this area so far required at least one stoichiometric additive, which is an obstruction for applications on scale.
Prof. Tobias Ritter and co-workers now developed a catalytic method for decarboxyolefination by employing photoredox catalysis and proton reduction catalysis in synergy. Abundant fatty acids can be converted to alpha olefins without any stoichiometric additives. In addition, this methodology can be applied on the late-stage functionalization of drug molecules and complex natural products. ‘Although this reaction cannot be used for manufacturing of olefins on a large scale, we are excited to report this conceptual way for sustainable alpha olefins synthesis. We think this work can hopefully serve as a stepping stone to further research in this area.’ Prof. Tobias Ritter said.
This work was described in detail in ‘Catalytic Dehydrogenative Decarboxyolefination of Carboxylic Acids’ published in Nature Chemistry on Oct. 8th 2018.
Figure: the simplified graph of reaction pathway.












