NMR-Based Structure Characterization
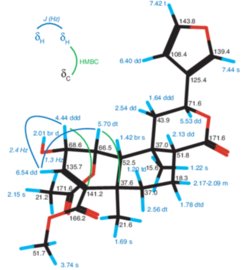
A reliable and efficient characterization of the constitution and configuration of chemical compounds is critical in essentially all areas of catalysis research. NMR plays a crucial role not only in the structural determination of reaction products but also in revealing spectroscopic fingerprints of the catalysts themselves. These spectroscopic identifications have proven essential to evaluate the performance, activity and selectivity of novel catalysts and to drive their further development. Indeed, NMR is routinely used for the characterization novel catalysts, in the evaluation of catalytic selectivities or for the rapid confirmation of each reaction product in a multi-step natural product synthesis campaign.
The NMR@kofo department has had a long tradition of providing independent and unbiased analysis of samples from all research groups over the last six decades. The department has specialized in the characterization of organometallic compounds, in assisting catalytic reaction scope evaluations as well as in the reliable confirmation of each product along novel natural product synthetic routes. Using state-of-the-art NMR methodologies and instrumentation, well over 100,000 compounds have been fully characterized over the years, delivering comprehensive multinuclear chemical shifts assignment along with scalar couplings and internuclear correlations. The technical know-how of our NMR staff in these areas rests on years of experience as well as on a vast in-house database.
Recent presentative publications:
- Zachmann, R. J.; Yahata, K.; Holzheimer, M.; Jarret, M.; Wirtz, C.; Fürstner, A. Total Syntheses of Nominal and Actual Prorocentin. Journal of the American Chemical Society 2023, 145, 2584–2595.
- Meng, Z.; Spohr, S. M.; Tobegen, S.; Farès, C.; Fürstner, A. A Unified Approach to Polycyclic Alkaloids of the Ingenamine Estate: Total Syntheses of Keramaphidin B, Ingenamine, and Nominal Njaoamine I. Journal of the American Chemical Society 2021, 143, 14402–14414.
- Löffler, L. E.; Wirtz, C.; Fürstner, A. Collective Total Synthesis of Casbane Diterpenes: One Strategy, Multiple Targets. Angewandte Chemie International Edition 2021, 60, 5316–5322.
- Hess, S. N.; Mo, X.; Wirtz, C.; Fürstner, A. Total Synthesis of Limaol. Journal of the American Chemical Society 2021, 143, 2464–2469.
- Schulthoff, S.; Hamilton, J. Y.; Heinrich, M.; Kwon, Y.; Wirtz, C.; Fürstner, A. The Formosalides: Structure Determination by Total Synthesis. Angewandte Chemie International Edition 2021, 60, 446–454.
- Heinrich, M.; Murphy, J. J.; Ilg, M. K.; Letort, A.; Flasz, J. T.; Philipps, P.; Fürstner, A. Chagosensine: A Riddle Wrapped in a Mystery Inside an Enigma. Journal of the American Chemical Society 2020, 142, 6409–6422.
