Enzyme catalysts with potential application in the production of new pharmaceuticals
Emeritus Prof. Reetz and collaborators report a fundamental study in Nature Communications
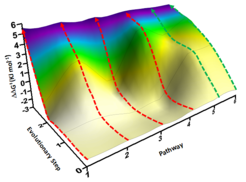
Figure: Excerpt of the P450 fitness pathway landscape featuring 2 energetically favored pathways (green) with no local minima and 4 disfavored pathways (red), all leading from starting enzyme to the same triple mutant (ΔΔG‡ are experimentally determined values).
It is well-known that upon introducing hydroxyl (OH) groups at certain positions in natural or synthetic steroids influences their biological activity. New applications in medicine are therefore possible, but it is a daunting task to develop efficient and general methods for ensuring regio- and stereoselectivity. Due to ecological and economical reasons, the formation of difficult to separate mixtures can no longer be tolerated.
In a 10-year transdisciplinary effort with 5 international groups, the basic question concerning, inter alia, the relationship between selectivity and activity of a biological catalyst in steroid hydroxylation was addressed experimentally and theoretically. In an earlier study, the Reetz group had genetically manipulated a cytochrome P450 monooxygenase as the catalyst for oxidatively introducing a hydroxyl group regio- and stereoselectively at, e.g., the 2ß-position with formation of a triple mutant having three additional mutational changes. This triple mutant formed the starting point of the new basic research study. The goal was the generation of information which would be useful in future more efficient targeted steroid hydroxylations.
First, all three single mutations of the triple mutant as well as all combinations thereof (deconvolution process) were produced as new biocatalysts and subsequently tested. Unexpected insights were indeed uncovered. Supported by quantum mechanical computations, unique views into P450-catalysis were revealed. The deconvolution process allowed, inter alia, the six theoretically possible mutational pathways leading from starting enzyme to the same triple mutant to be identified in the form of a „fitness pathway landscape“ (see Figure below). The Reetz group had developed such a deconvolution procedure some years ago for mechanistic purposes, which was now refined and applied for the first time to the difficult P450-catalyzed steroid-hydroxylation. This study also sheds light on the relationship between enzyme selectivity and activity.
The results of this basic research will be of significant help in generating future P450 mutants for targeting steroid hydroxylation at essentially any position, including the hotly sought C7-selectivity.
Here you can find the link to the article "Pervasive cooperative mutational effects on multiple catalytic enzyme traits emerge via long-range conformational dynamics"











