Easy access to corundum (α-Al2O3) in nanoparticulate form – a material of high academic and industrial interest
Corundum (a-Al2O3, aluminum oxide in alpha phase) is technically one of the most important mineral phases. If available in nanoparticulate form, it could lead to step changes in its existing uses and enable new applications. For instance, in auto-exhaust catalysis, it could replace gamma alumina and can be used directly as support – thanks to the high mechanical stability of the alpha structure and high surface area offered by the nanocrystalline form. In ceramic applications such as dental prostheses and high-speed cutting tools, it would allow the production of ceramics with improved fracture toughness and high density at reduced sintering temperatures, thereby enabling energy saving.
However, its formation from cubic close-packed oxides (transition alumina) requires to overcome a high activation energy barrier, which is conventionally done by the application of high temperatures (above 1200°C) which in turn leads to sintering and severe loss in surface area.
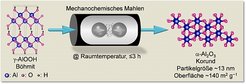
Ferdi Schüth and co-workers now found a way to produce nanometer-sized-α-Al2O3 (particle diameter ~13 nm, surface area ~140 m2 g−1) simply by mechanochemical dehydration of boehmite (α-AlOOH) at room temperature. This transformation, which is different from a normal chemical reaction, proceeds via microstructural rearrangements during the mechanical grinding in the ball mill, explains Ferdi Schüth, who supervised the project at the Max-Planck-Institut für Kohlenforschung. Depending on the grinding conditions, a minor amount of rare mineral phases such as diaspore and tohdite may occur as intermediates, says Schüth.
The mechanochemical approach has also been attempted before to convert gamma alumina to alpha alumina. However, associated microstructural rearrangements in gamma alumina precursor led to a severe loss in surface area and the resultant alpha alumina had 2-3 times lower surface area than its precursor. The choice of boehmite precursor in our work was the crucial step to achieve nanocrystalline alpha alumina, which has a surface area much higher than its precursor, explains Amol Amrute, who led the project activities at the Max-Planck-Institut für Kohlenforschung. The boehmite precursor has structural water (one mole per mole of alumina), which is released upon dehydration of boehmite to corundum during milling. This released water plays a decisive role:
(1) in breaking down the agglomerate formed during initial stages of milling into nanoparticle form and
(2) in stabilizing the formed corundum nanoparticles by hydroxylation, says Amrute.
Thermodynamic calculations, performed by Zbigniew Łodziana at INP, Polish Academy of Sciences, indicate that this transformation is driven by a shift in the stability from boehmite to α-Al2O3 caused by milling impacts on the surface energy. The hydroxylation of corundum nanoparticles further shifts the stability towards α-Al2O3, making it the most stable form under the process conditions.
The new process has many advantages over previous manufacturing processes as it can be performed by using an easily accessible boehmite precursor at room temperature and requires only a few hours. Besides, this process is very flexible towards the choice of the precursor, as a boehmite sample with 10 m2 g−1 initial surface area can also lead to corundum with surface area as high as 130 m2 g−1. Thus, nanoparticulate corundum with high specific surface areas is easily accessible now.
The results have been published on October 25 in Science: "High-surface-area corundum by mechanochemically induced phase transformation of boehmite".
figure: Boehmite, a water-containing aluminium oxide, is used to produce corundum nanoparticles with a surface area of 140 square metres per gram when ground for about three hours. © Amol Amrute, MPI für Kohlenforschung












