Reaction intermediates
A glance into the catalytic pathway from substrate to products offers valuable insight into its reaction mechanism. Unfortunately, the enlightening reaction intermediates are typically short-lived and at low-concentration, so that their characterisation by NMR can be challenging. Several strategies can be adopted to detect these: (1) making use of state-of-the-art instrumentation and/or special experiments which can boost the sensitivity of the NMR detection or (2) using chemical strategies to promote and stabilise intermediates, so that they become long-lived on the NMR timescale. Here are some representative accomplishments:
Detection of gem-hydration carbene intermediate with PHIP: The inherently poor sensitivity of NMR comes from the fact that the signal originates from the thermal polarisation of nuclear spins inside a strong magnetic field. There exist however other possible sources of NMR polarisation. For instance, when molecular hydrogen in its stable para-spin state (para-H2) is transferred pairwise to a molecule, e.g. in the hydrogenation reaction of a triple bond, the symmetry of the molecule is broken while the anti-parallel spin orientation is preserved. In effect, the para-H2 “stores” 100% polarisation which can be “released” via its transfer onto a molecule leading to a theoretical 10000x boost in sensitivity. This method (para-Hydrogen Induced Polarization or PHIP) was used for the study of short-lived and low-concentration reaction intermediates. It has been used in our laboratory in the mechanistic study of Ru-catalysed trans-hydration of alkyne.
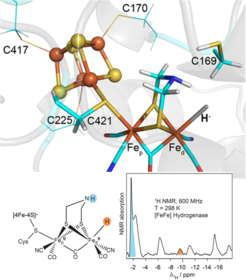
Detection of the Terminal Hydride Intermediate in [FeFe] Hydrogenase: Using high-sensitivity solution 1H NMR spectroscopy at room temperature, strongly paramagnetically broadened and shifted 1H signals could be detected and assigned to key hydrogen positions in the iron-rich clusters of the [FeFe] hydrogenase from Chlamydomonas reinhardtii in different states of the hydrogen-producing catalytic cycle. Among these, an intermediate state involving a terminal iron-bound hydride, recognized as crucial for the catalytic mechanism, was directly detected, thus proving its occurrence unequivocally at physiological conditions for the first time.
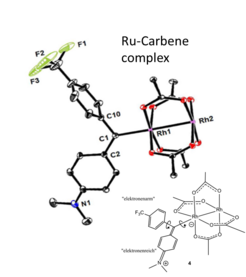
Stabilization and Structural Study of a Chiral Dirhodium Carbene Intermediate: For over a decade, the remarkable catalytic properties of the bimetallic dirhodium “paddlewheel” complex to activate C-H bond and used in asymmetric cyclopropanation, cycloaddition and ylid formation have been resting on a key Rh-carbene intermediate which was never before detected due to its known instability. In complement to the valuable crystallographic information on stabilized Rh-carbene intermediates, NMR could not only confirm that structural features such as the position and orientation of the carbene ligand are also present in solution but also could deliver a detailed dynamic portrait essential for the understanding of the reaction mechanism.
Representative publications:
[1] D. J. Tindall, C. Werle, R. Goddard, P. Philipps, C. Farès, A. Fürstner, J. Am. Chem. Soc. 2018, 140, 1884-1893.
doi: http://doi.org/10.1021/jacs.7b12673
[2] C. Sommer, S. Rumpel, S. Roy, C. Farès, V. Artero, M. Fontecave, E. Reijerse, W. Lubitz, J. Biol. Inorg. Chem. 2018, 23, 481-491.
doi: http://doi.org/10.1007/s00775-018-1558-4
[3] S. Rumpel, C. Sommer, E. Reijerse, C. Farès, W. Lubitz, J. Am. Chem. Soc. 2018, 140, 3863-3866.
doi: http://doi.org/10.1021/jacs.8b00459
[4] S. Rumpel, E. Ravera, C. Sommer, E. Reijerse, C. Farès, C. Luchinat, W. Lubitz, J. Am. Chem. Soc. 2018, 140, 131-134.
doi: http://doi.org/10.1021/jacs.7b11196
[5] A. Guthertz, M. Leutzsch, L. M. Wolf, P. Gupta, S. M. Rummelt, R. Goddard, C. Farès, W. Thiel, A. Fürstner, J. Am. Chem. Soc. 2018, 140, 3156-3169.
doi: http://doi.org/10.1021/jacs.8b00665
[6] C. Werlé, R. Goddard, P. Philipps, C. Farès, A. Fürstner, Angew. Chem. Int. Ed. 2016, 55, 10760-10765.
doi: http://doi.org/10.1002/anie.201605502
[7] C. Werlé, R. Goddard, P. Philipps, C. Farès, A. Fürstner, J. Am. Chem. Soc. 2016, 138, 3797-3805.
doi: http://doi.org/10.1021/jacs.5b13321
[8] M. Leutzsch, C. Farès, MPI Kohlenforschung Forschungsbericht 2016 2016. doi:
[9] M. Leutzsch, L. M. Wolf, P. Gupta, M. Fuchs, W. Thiel, C. Farès, A. Fürstner, Angew. Chem. Int. Ed. 2015, 54, 12431-12436.
doi: http://doi.org/10.1002/anie.201506075


