Novel Concepts for Catalysis
Various lines of research are pursued by which we intend to establish new principles for catalysis and to develop conceptually novel ligand architectures.
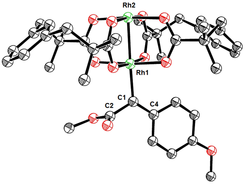
Carbene Chemistry. The formation of “super-electrophilic” carbene complexes by decomposition of diazoalkanes with the help of (chiral) dirhodium tetracarboxylate complexes is a well-established methodology for (asymmetric) catalysis. For their exceptional reactivity, however, intermediates of this type basically defied experimental characterization for decades. Our group managed for the first time to characterize a set of representative rhodium carbenes of this and related types by X-ray crystallography and NMR.
These data allow many results from the literature to be rationalized and new concepts for catalyst design to be explored. Among them is the development of new heteroleptic as well as heterobimetallic complexes for enantioselective catalysis. The development of an unprecedented rhodium-catalyzed metathesis of azobenzene derivatives also deserves mentioning in this context.
Additional information can be found in the following presentation.
Metal Carbene Complexes
Contributions of the Fürstner Laboratory
Redox Catalysis. We are striving to replace notoriously stoichiometric reactions of proven versatility by catalytic processes. A particularly successful example is the development of Nozaki-Hiyama-Kishi (NHK) reactions catalytic in chromium, which paved the way to asymmetric catalysis and even found industrial applications. Likewise, we were able to develop the first carbonyl coupling reactions catalytic in titanium; they opened a new entry into indoles and other heterocycles.
Additional information can be found in the following presentation.
The First Nozaki-Hiyama-Kishi Reactions Catalytic in Cronium
Cross Coupling. Our group has introduced the “9-methoxy-9-BBN variant” of the Suzuki reaction as a highly versatile and base-free procedure for cross coupling. Moreover, an improved method for Stille-Migita reactions was described, which is applicable to polyfunctionalized and sensitive substrates and therefore became quite popular in the community.
Additional information can be found in the following presentation.
Improved Protocol For Stille Coupling Reactions
Coordination Chemistry at Carbon. Organic molecules are commonly understood as substances based on carbon atoms, which involve all four valence electrons in bonding. If two electrons form a lone-pair, one crosses the traditional borders to coordination chemistry and/or enters the realm of reactive intermediates. Compounds, wherein a carbon atom configures all four valence electrons in form of two lone-pairs may seem elusive at first sight. Following insightful early reports and recent computational studies, however, our group has studied the preparation and reactivity of compounds, in which carbon serves as the “central atom” of a “complex”. The description of certain carbogenic materials as coordination compounds differs fundamentally from the common understanding of organic chemistry.
Additional information can be found in the following presentation.
