Publikationen (Zusammenfassung): Teil 2
Nr. 41 - 22
Publikationen (Zusammenfassung): Teil 1
41. Synthesis of Sulfonyl Fluorides from Sulfonamides
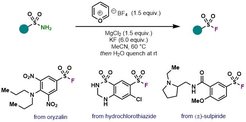
Perez-Palau, M.; Cornella, J. Eur. J. Org. Chem. 10.1002/ejoc.202000022
40. An Air-Stable Binary Ni(0)-Olefin Catalyst

Nattmann, L; Saeb, R.; Nöthling, N.; Cornella, J. Nature Catalysis 2020, 3, 6-13
-
Highlighted in Nature Catalysis, News and Views, 2019 (Boit, T. B.; Spence, K. A.; Garg, N.K.)
-
Highlighted in C&EN (Nickel catalyst fends off air attack).
-
Highlighted in Org. Process. Res. Dev. 2020. doi: 10.1021/acs.oprd.0c00027.
39. Radical C-N Borylation of Aromatic Amines Enabled by a Pyrylium Reagent.
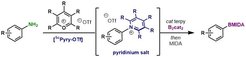
Ma, Y.; Pang, Y.; Chabbra, S.; Reijerse, E. J.; Schnegg, A.; Niski, J.; Leutzsch, M.; Cornella, J. Chem. -Eur. J. 2020, 26, 3738-3743.
Previously in ChemRxiv 2019, doi: 10.26434/chemrxiv.11211245.v1
38. Pyrylium Salts: Selective Reagents for the Activation of Primary Amino Groups in Organic Synthesis

Pang, Y.; Moser, D. Cornella, J. SYNTHESIS 2020, 52, 489-503. (Part of the Bürgenstock Special Edition 2019 Future Stars in Organic Chemistry)
37. Facile Access to Chiral non-Natural Aminoacids
Planas, O.; Cornella, J. Nature Catalysis 2019, 2, 839. (News & Views Article)
36. Selective Late-Stage Sulfonyl Chloride Formation from Sulfonamides Enabled by Pyry-BF4
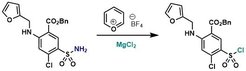
Gomez-Palomino, A.; Cornella, J. Angew. Chem. Int. Ed. 2019, 58, 18235-18239
-
Highlighted in Organic Process Research and Development (OPR&D) Org. Process Res. Dev. 2019, 23, 2583−2591
35. Fluorination of Arylboronic Esters Enabled by Bismuth Redox Catalysis

Planas, O.; Wang, F.; Leutszch, M.; Cornella, J. Science, 2020, 367, 313-317.
Previously in ChemRxiv (doi.org/10.26434/chemrxiv.9729143.v1)
-
Highlighted in C&EN (Bismuth, often overlooked in the periodic table, breaks into redox catalysis).
-
Highlighted in Organic Process Research and Development (OPR&D) 2020, 24, 323-333
-
Highlighted in Synfacts 2020, 16, 409.
34. Bi(I)-catalyzed Transfer-Hydrogenation with Ammonia Borane
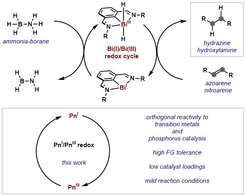
Wang, F.; Planas, O.; Cornella, J. J. Am. Chem. Soc. 2019, 141, 4235
-
Highlighted in Chemistry Views - News March 2019.
-
Highlighted in ChemBeanGo - April 2019 (available in chinese).
-
Most read article of the month J. Am. Chem. Soc. (March 2019).
33. Ni-catalyzed Reductive Liebeskind-Srogl Alkylation of Heterocycles
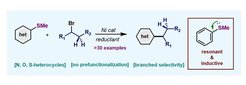
Ma, Y.; Cammarata, J.; Cornella, J. J. Am. Chem. Soc. 2019, 141, 1918
-
Most read article of the month in J. Am. Chem. Soc. (January 2019)
-
Highlighted in Synfacts, 2019, 15, 379
-
Highlighted in Org. Process Res. Dev. 2019, 23, 289.
32. A Highly Reduced Ni-Li-Olefin Complex for Kumada-Corriu Cross-Couplings
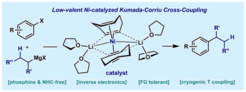
Nattmann, L.; Lutz, S.; Ortsack, P.; Goddard, R. Cornella, J. J. Am. Chem. Soc. 2018, 140, 13628
31. A Perspective in Catalysis: Development of Efficient Methods in the Age of Sustainability
O'Neill, M. J.; Cornella, J. CHIMIA, 2018, 72, 601.
30. Retaining Alkyl Nucleophile Regiofidelity in Transition-Metal-Mediated Cross-Couplings to Aryl Electrophiles
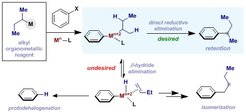
O'Neill, M. J.; Cornella, J. SYNTHESIS, 2018, 50, 3974.
29. Selective Functionalization of Aminoheterocycles by a Pyrylium Salt
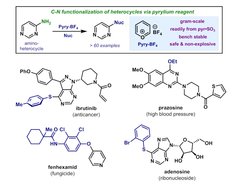
Moser, D.;* Duan, Y.;* Wang, F.; Ma, Y.; O'Neill, M. J.; Cornella, J. Angew. Chem. Int. Ed. 2018, 57, 11035.
-
Most Accessed Article in Angewandte Chemie Internationat Edition (July 2018)
-
Highlighted in SynFacts, 2018, 14, 1123
28. Thorpe-Ingold Effect for Branch-Selective Alkylation of Unactivated Aryl Fluorides
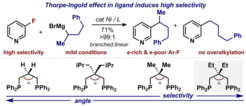
O’Neill, M. J.;* Riesebeck, T.;* Cornella, J. Angew. Chem. Int. Ed. 2018, 57, 9103 (* equal contribution)
-
Highlighted in SynFacts, 2018, 14, 967
Prior to MPI
27. CITU: A Peptide and Decarboxylative Coupling Reagent.
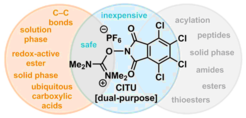
Justine N. deGruyter, Lara R. Malins, Laurin Wimmer, Khalyd J. Clay, Javier Lopez-Ogalla, Tian Qin, Josep Cornella, Zhiqing Liu, Guanda Che, Denghui Bao, Jason M. Stevens, Jennifer X. Qiao, Martin P. Allen, Michael A. Poss, and Phil S. Baran. Org. Lett. 2017, 19, 6196.
26. Remote Carboxylation of Halogenated Aliphatic Hydrocarbons with Carbon Dioxide
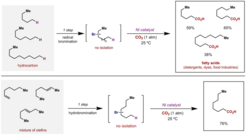
Juliá-Hernandez, F.; Moragas, T.; Cornella, J; Martin, R. Nature, 2017, 545, 84.
25. Alkyl-(Hetero)Aryl Bond Formation via Decarboxylative Cross-Coupling: A Systematic Analysis.

Sandfort, F.;* O’Neill, M. J.;* Cornella, J.; Wimmer, L.; Baran, P. S. Angew. Chem. Int. Ed. 2017, 56, 3319.
24. Visible-Light-Promoted Atom Transfer Radical Cyclization of Unactivated Alkyl Iodides.

Y. Shen; Cornella, J.; F. Julia-Hernandez; Martin, R. ACS Catal., 2017, 7, 409.
23. Redox-Active Esters in Fe-catalyzed Cross-Coupling
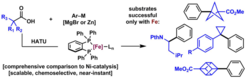
Toriyama, F.;* Cornella, J.;* Wimmer, L.; Chen, T. –G.; Dixon, D. D.; Creech, G.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 11132.
22. Nickel-catalyzed Cross-coupling of Redox-Active Esters with Boronic Acids.

Wang, J.; Qin, T.; Chen, T. –G.; Wimmer, L.; Edwards, J. T.; Cornella, J.; Vokits, B.; Shaw, S. A.; Baran, P. S. Angew. Chem. Int. Ed. 2016, 55, 9676.
- VIP paper (Very Important Paper)
- Most accessed article of the year 2016 in Angewandte Chemie International Edition.

















