Publikationen (Zusammenfassung): Teil 1
Nr. 21 - Nr. 1
Publikationen (Zusammenfassung): Teil 2
21. A General Alkyl-Alkyl Cross-Coupling Enabled by Redox-Active Esters and Alkylzinc Reagents.
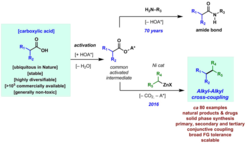
Qin, T.;* Cornella, J.;* Li, C.;* Malins, L. R.; Edwards, J. T.; Kawamura, S.; Maxwell, B. D.; Eastgate, M. D.; Baran, P. S. Science. 2016, 352, 801.
- Highlighted by Chemistry World, April 2016.
- Highlighted in Angew. Chem. Int. Ed. 2016, 55, 11340.
- Highlighted in Synfacts, 2016, 12, 723.
20. Practical Ni-Catalyzed Aryl-Alkyl Cross-Coupling of Secondary Redox-Active Esters.
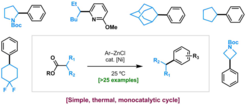
Cornella, J.;* Edwards, J. T;* Qin, T.; Kawamura, S.; Wang, J.; Pan, C. –M.; Gianatassio, R.; Schmidt, M.; Eastgate, M D.; Baran, P. S. J. Am. Chem. Soc. 2016, 138, 2174.
- Highlighted in Angew. Chem. Int. Ed.
- Highlighted in Angew. Chem. Int. Ed. 2016, 55, 11340.
- Most Read Article of the year 2016 in Journal of the American Chemical Society.
19. Ni-catalyzed Enantioelctive C–C Bond Formation via C(sp2)–O Cleavage in Aryl Esters.

Cornella, J. Jackson, E. P.; Martin, R. Angew. Chem. Int. Ed. 2015, 54, 4075.
- Highlighted in Synfacts 2015, 11, 389.
18. Ligand-controlled Regiodivergent Ni-catalyzed Reductive Carboxylation of Allyl Esters with CO2.
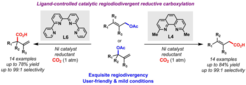
Moragas, T.;* Cornella, J.;* Martin, R. J. Am. Chem. Soc. 2014, 136, 17702.
17. Ni-catalyzed Carboxylation of Unactivated Primary Alkyl Bromides and Sulfonates with CO2.

Liu, Y.; Cornella, J.; Martin, R. J. Am. Chem. Soc. 2014, 136, 11212.
16. Metal-catalyzed Activation of Ethers via C–O Bond Cleavage: A New Strategy for Molecular Diversity.

Cornella, J.; Zarate, C.; Martin, R. Chem. Soc. Rev. 2014, 43, 8081.
15. Ni-catalyzed Reductive Cleavage of Methyl 3-Methoxy-2-Naphthoate.

Cornella, J.; Zarate, C.; Martin, R. Org. Synth. 2014, 91, 60.
14. Ni-catalyzed Stereoselective Arylation of Inert C–O bonds at Low Temperatures.

Cornella, J.; Martin, R. Org. Lett. 2013, 15. 6298.
- Highlighted in Synfacts 2014, 10, 296.
13. A Combined Experimental and Theoretical Study on the Reductive Cleavage of Inert C–O Bonds with Silanes: Ruling out a Classical Ni(0)/Ni(II) Catalytic Couple and Evidence for Ni(I) Intermediates.
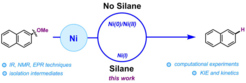
Cornella, J.; Gomez-Bengoa, E.; Martin, R. J. Am. Chem. Soc. 2013, 135, 1997.
12. Nickel-Catalyzed Decarbonylative C–H Coupling Reactions: A Strategy for Preparing Bis(Heteroaryl) Backbones (Highlight)
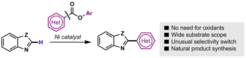
Correa, A.; Cornella, J.; Martin, R. Angew. Chem. Int. Ed. 2013, 52, 1878.
11. The ortho-Substituent Effect on the Ag-catalysed Decarboxylation of Benzoic Acids.

Grainger, R.; Cornella, J.; Blakemore, D. C.; Larrosa, I.; Campanera, J. –M. Chem. –Eur. J. 2014, 20, 16680.
10. Selective Deuteration of (Hetero)aromatic Compounds via Deutero-decarboxylation of Carboxylic Acids.

Grainger, R.; Nikmal, A.; Cornella, J.; Larrosa, I. Org. Biomol. Chem. 2012, 10, 3172.
9. Decarboxylative C–C Bond Forming Transformations of (Hetero)aromatic Carboxylic Acids.
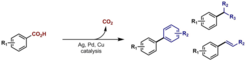
Cornella, J.; Larrosa, I. Synthesis, 2012, 44, 653.
8. Carboxylic Acids as Traceless Directing Groups for Formal meta-Selective Direct Arylation.
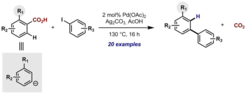
Cornella, J.; Righi, M.; Larrosa, I. Angew. Chem. Int. Ed. 2011, 40, 9429.
7. A Novel Mode of Reactivity for Gold(I): The Decarboxylative Activation of (Hetero)aromatic Carboxylic Acids.
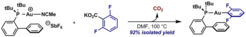
Cornella, J.; Rosillo-Lopez, M.; Larrosa, I. Adv. Synth. Catal. 2011, 353, 1359.
6. Stereodivergent Addition of 4-Sililoxy-1,2-Allenes to Aldehydes.
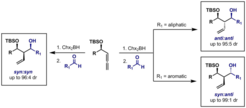
Sanchez, C.; Ariza, X.; Cornella, J.; Farras, J.; Garcia, J. Chem. –Eur. J. 2010, 16, 11535.
5. Decarboxylative Homocoupling of (Hetero)aromatic Carboxylic Acids.
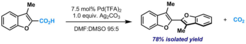
Cornella, J.; Lalhali, H.; Larrosa, I. Chem. Commun. 2010, 46, 8276.
4. Silver-Catalyzed Protodecarboxylation of Heteroaromatic Carboxylic Acids.

Lu, P. F.; Sanchez, C.; Cornella, J.; Larrosa, I. Org. Lett. 2009, 11, 5710.
- Highlighted in Synfacts 2010, 3, 0338.
3. Intermolecular Decarboxylative Direct C-3 Arylation of Indoles with Benzoic Acids.

Cornella, J.; Lu, P. F.; Larrosa, I. Org. Lett. 2009, 11, 5506.
- Highlighted in Org. Process. Res. Dev. 2010, 14, 300.
2. Silver-catalysed Protodecarboxylation of ortho-Substituted Benzoic Acids.

Cornella, J.; Sanchez, C.; Banawa, D.; Larrosa, I. Chem. Comm. 2009, 46, 7176.
1. Stereocontrolled Synthesis of Highly Functionalized Quaternary Carbon Centers: A Route to alpha-Substituted Serines.

Ariza, X.; Cornella, J.; Font-Bardia, M.; Garcia, J.; Ortiz, J.; Sanchez, C.; Solans, X. Angew. Chem. Int. Ed. 2009, 48, 4202.




















