trans-Hydrogenation, gem-Hydrogenation und trans-Hydrometalation
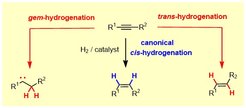
During the course of a catalytic hydrogenation, both H-atoms of H2 are transferred to the same side of the p-system of a given substrate (“syn”/”suprafacial”). This paradigm remained basically unchallenged since the time when catalytic hydrogenation was discovered over a century ago. Our group managed to show that this stereochemical rule can easily be overcome with the aid of simple ruthenium catalysts, which allow internal alkynes to be reduced to E-alkenes with high levels of selectivity.
Detailed mechanistic studies proved that this unconventional “trans-hydrogenation” is linked to yet another, even more unorthodox transformation, during which both H-atoms are transferred to one and the same C-atom of the alkyne: such geminal hydrogenation reactions of stable carbogenic substrates are without precedent. The fact that they lead to the concomitant formation of discrete metal carbene complexes opens unexpected new opportunities: “hydrogenative enyne metathesis”, “hydrogenative cyclopropanations” as well as “hydrogenative heterocycle syntheses” are representative examples.
Moreover, we were able to show that trans-hydrogenation is by no means a singularity: rather, conventional hydroboration as well as hydrostannation (silylation, germylation) can also be morphed into non-canonical trans-addition processes; once again, this stereochemical course clearly violates textbook knowledge.
Investigations into the underlying mechanisms, a further extension of this highly unorthodox reactivity mode, and the implementation of the new reactions into target-oriented synthesis are focal points of our current research activities.
Additional information can be found in the following presentation.
