Cationic Ligands: Synthesis and Applications of Extreme π-Acid Catalysts
The goal of this project is the synthesis of extreme π-acceptor phosphines through the introduction of positively charged homo- or heteroaromatic substituents directly attached to the phosphorus atom. By exploiting this property, new Au and Pt catalysts have been developed that display a dramatically enhanced capacity to activate π-systems.
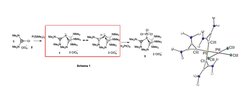
Very recently, we reported the synthesis of the first ever isolated carbene-stabilized P1-centered trication [L3P]3+ (L = 2,3-dialkylaminocyclopropenium) 1 by reaction of the 1-chloro-2,3-(dimethylamino) cyclopropenium salt 2 and P(SiMe3)3 (Scheme 1).
Despite the three positive charges on the groups directly attached to the P atom, this compound can still serve as a ligand for π-acidic metals such as Pt. Thus, when 1 is treated with K2PtCl4 in acetonitrile, the bench stable complex 3 is formed. More interestingly, charge decomposition analysis of the metal-ligand interaction in 3 gave the surprising result that the total L→M σ-donation (0.31 e) is lower than the L→M π-back donation (0.43 e) into the very low-lying LUMO of 1, which must hence be regarded as the main interaction in 3. This unconventional situation in which the P-ligand removes net electron density from the metal suggests that compound 1 increases the natural π-acidity of Pt(II) centers. It should thus accelerate known reactions, or even permit new ones, in which either the coordination of the substrate or the nucleophilic attack to the activated substrate are the rate-determining steps.
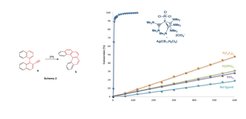
Accordingly, we chose for our studies on ligand effects a series of phosphanes such as PPh3, P(OPh)3, P(C6F5)3 and precatalyst 3 in combination with a silver salt. As expected, both P(OPh)3 and P(C6F5)3 performed better than PPh3 in terms of reactivity (Scheme 2).

In addition, our synthetic program has already benefitted from these novel tools and natural products such as Orchinol, Ochrolide, Bulbophyllantrin and Epimedoicarisoside A have been prepared using our Pt and Au catalysts for the key hydroarylation step (Scheme 3).


