Interfaces
“Liquid|liquid interfaces (LLIs) strike our mind not only with every oil catastrophe but also when thinking about many home-cooked and industrial food products. There are important exchange processes through these interfaces determining degradation and molecular uptake processes. Also, in biology, these underestimated interfaces start to fascinate researchers after the recovery of the membrane-less cell organelles. Why has nature chosen this way of dividing for functional units? What is the consequence of it on exchange processes in the cell? Surprisingly, there is not much textbook knowledge about these kinds of interfaces.”(1)
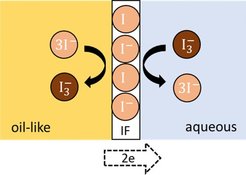
In an experimental work(1), we investigate Liquid|liquid interfaces between redox-type electrolytes with a 2-electrode technique by the subtraction of the volume effects of the adjacent bulk phases and a correction of equipment errors. In this system, the electron transfer through the LLI turns out to overdominate the ion transfer. Surprisingly, no indication for a barrier in the charge transfer was found. The measured resistances assignable to the LLI are extremely low. The efficient charge transfer through the LLI is ascribed to an ionic adsorption layer acting as a buffer for the electrons and opening a new channel for the charge transport. The impedance spectra show a specific peculiarity: a “capacitance lag” at high frequencies. This lag is interpreted using a new model of delayed transport. Microscopically, such a kind of transport is explained with an adsorbed ion layer with variable charge. It acts as a buffer and causes a time delay in the charge transfer.
(1) J. Akilavasan, F. Marlow: Electrochemical Impedance Spectroscopy at Redox-type Liquid|Liquid Interfaces: The Capacitance Lag. The Journal of Physical Chemistry C 124 (2020) 4101−4108.
http://dx.doi.org/10.1021/acs.jpcc.9b10116.
