A new catalytic method for the synthesis of amines. Bill Morandi and his team report on a simple iron-catalyzed reaction that leads to unprotected primary amines
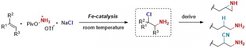
Primary amines are essential components of biologically active molecules and versatile intermediates in the synthesis of drugs and agrochemicals. However, their preparation from easily accessible alkenes remains a challenge and has so far only been possible through the use of "protective groups". Bill Morandi and his team have now developed a new general strategy that offers access to primary amines from alkenes via a functionally simple iron-catalyzed amino chlorination reaction. It works through the addition of unprotected NH2 from a hydroxylamine derivative directly to a double-bonded carbon center in the presence of table salt (NaCl) as a chloride source. The resulting novel amine derivatives 2-chloroalkylamines have a high reactivity and can be easily converted into a multitude of different, useful amines in asubsequent step. The special feature of the reaction is that it now takes place without "protective groups" and is based on a simple iron catalyst, which fully meets the desire for sustainability.
Bill Morandi's team started research at the amino groups at the Max-Planck-Institut für Kohlenforschung, where Bill Morandi worked as an independent research group leader in the Department of Homogeneous Catalysis until July of this year. In the meantime, the 35-year-old has been appointed at the ETH Zurich as professor at the Laboratory of Organic Chemistry. The article "Efficient access to unprotected primary amines by iron-catalyzed aminochlorination of alkenes" was published on 26 October 2018 in Science.












