NMR-based 3D Structural models
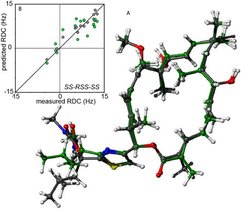
Compared to crystallography which provides exact atomic coordinates of compounds in specific solid-state stable forms, NMR affords “looser” atomic-scale structural information yet these can be obtained under almost any conceivable conditions (temperature, pressure, solvent, mixtures, etc.). The typically time- and space-averaged information provided by nuclear spin interactions such as cross-relaxation (nOes), couplings, chemical shifts and quadrupolar couplings are typically short-range, inexact and empirically evaluated. Yet a rich dataset of carefully evaluated spin interactions can be applied as restraints for guiding ensemble simulations and provide instructive 3d-models of molecules.
Orientation-based interactions such as Residual Dipolar Couplings (RDC) and Residual Chemical Shift Anisotropies (RCSAs) are considered “long-range” NMR-based information and complement the classical short-range NMR information provided by nOes and scalar couplings. They can be used to determine stereochemistries, to differentiate enantiomers and to provide conformational and dynamic information.
Developments are ongoing in many aspects of 3d-model determination including sample preparation (orienting media), NMR-data measurement and evaluation as well as theoretical ensemble simulations using classical (MD) and quantum (DFT) methods.
Representative publications:
[1] H. Kim, G. Gerosa, J. Aronow, P. Kasaplar, J. Ouyang, J. B. Lingnau, P. Guerry, C. Fares, B. List, Nat Commun 2019, 10.
doi: http://doi.org/10.1038/s41467-019-08374-z
[2] C. Fares, J. B. Lingnau, C. Wirtz, U. Sternberg, Molecules 2019, 24.
doi: http://doi.org/10.3390/molecules24234417
[3] T. Niklas, P. Schulze, C. Fares, Magn. Reson. Chem. 2018, 56, 1176-1182.
doi: http://doi.org/10.1002/mrc.4786
[4] O. Lifchits, M. Mahlau, C. M. Reisinger, A. Lee, C. Farès, I. Polyak, G. Gopakumar, W. Thiel, B. List, J. Am. Chem. Soc. 2013, 135, 6677-6693.
doi: http://doi.org/10.1021/Ja402058v
[5] C. Farès, J. Hassfeld, D. Menche, T. Carlomagno, Angew. Chem. Int. Ed. 2008, 47, 3722-3726.
doi: http://doi.org/10.1002/anie.200800225
