Late-Stage Functionalization
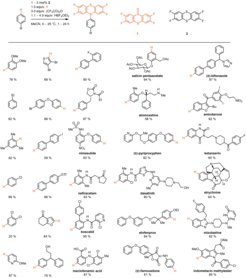
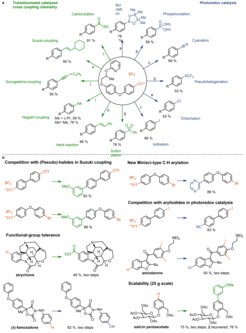
The term late-stage functionalization (LSF) is now frequently used in the field of organic methodology development to describe transformations on complex molecules. Such reactions include catalytic and non-catalytic reactions, C–H functionalizations, and functional group manipulations, with one or several desired products. My group has contributed to the development of LSF in diverse areas such as drug development and molecular imaging. We define LSF as a desired chemical transformation on a complex molecule, without the necessity for a preinstalled functional group that exclusively serves the purpose to enable the transformation. LSF of a complex molecule can access at least one new, desired, functionalized molecule that would be substantially more cumbersome or time-consuming to access synthetically otherwise. As such, LSF can quickly provide access to molecules of potential value, for example in the area of drug development or materials chemistry, that otherwise may not have been available at all, or too burdensome to make. Our most powerful LSF as of yet is the exquisitely selective arene C–H functionalization to aryl sulfonium salts that present synthetically useful electrophiles for subsequent transformations. The method is unusual in the sense that for most arenes, independent of substitution pattern and directing groups, a single constitutional isomer, within the limits of conventional detection, of the functionalized product can be isolated. Subsequently, the sulfonium-based linchpin can be used as a useful synthetic handle in C–C, C–N, C–O, C–S, and C–F bond-forming reactions. We have shown for the first time that the specific arylsulfonium salts based on the heterocycle thianthrene are excellent substrates for conventional palladium-catalyzed cross-coupling chemistry, as well as provide conceptual advances in photoredox-catalyzed transformations when compared to other aryl (pseudo)halides. The sulfonium-based C–H functionalization reaction is currently the most selective aryl C–H functionalization reaction available that does not require a specific directing group or substitution pattern.
Select publications:
F. Berger, M. B. Plutschack, J. Riegger, W. Yu, S. Speicher, M. Ho, N. Frank, T. Ritter “Site-selective and versatile aromatic C−H functionalization by thianthrenation“ Nature 2019, 567, 223–228.
DOI: 10.1038/s41586-019-0982-0
P. Xu, D. Zhao, F. Berger, A. Hamad, J. Rickmeier, R. Petzold, M. Kondratiuk, K. Bohdan, T. Ritter “Site-selective late-stage aromatic 18F-fluorination via aryl sulfonium salts“ Angew. Chem. Int. Ed. 2020, 59, 1956–1960.
J. Li, J. Chen, R. Sang, W.S. Ham, M. B. Plutschack, F. Berger, S. Chabbra, A. Schnegg, C. Genicot, T. Ritter “Photoredox catalysis with aryl sulfonium salts enables site-selective late-stage fluorination“ Nature Chem. 2019, 12, 56–62.
DOI: 10.1038/s41557-019-0353-3
R. Sang, S. Korkis, W. Su, F. Ye, P. S. Engl, F. Berger, T. Ritter “Site‐selective C−H oxygenation via aryl sulfonium salts“ Angew. Chem. Int. Ed. 2019, 58, 16161–16166.
P. S. Engl, A. P. Häring, F. Berger, G. Berger, A. Pérez-Bitrián, T. Ritter “C–N cross-couplings for site-selective late-stage diversification via aryl sulfonium salts“ J. Am. Chem. Soc. 2019, 141, 13346–13351.
F. Ye, F. Berger, H. Jia, J. Ford, A. Wortman, J. Börgel, C. Genicot, T. Ritter “Aryl sulfonium salts for site‐selective late‐stage trifluoromethylation“ Angew. Chem. Int. Ed. 2019, 58, 14615–14619.