Molekulare Theorie und Spektroskopie
Unseren Abteilung beschäftigt sich mit der Grundlagenforschung im Zusammenhang mit der Aktivierung kleiner Moleküle durch Übergangsmetalle im weitesten Sinne sowie mit der Entwicklung und Anwendung von Methoden der Quantenchemie. Die Aktivitäten der Gruppe umfassen drei große, miteinander verknüpfte Bereiche:
I. Entwicklung neuer quantenchemischer Methoden
II. Computerchemie
III. Molekulare Spektroskopie
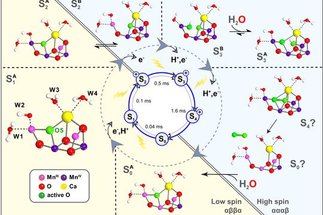
Das übergeordnete Ziel der Abteilung ist es, komplexe Reaktionsmechanismen der Übergangsmetallkatalyse auf der Ebene der Elektronenstruktur zu entschlüsseln. Da die experimentellen Methoden zur Untersuchung der Elektronenstruktur zahlreiche Arten der Spektroskopie umfassen, ist ein grundlegendes Verständnis der Zusammenhänge von Struktur und Spektren von zentraler Bedeutung. Auch die Charakterisierung von wichtigen Reaktionsintermediaten kann praktisch immer ausschließlich durch die Interpretation von Spektren erfolgen, die transient oder über quenching Techniken erhalten wurden.
Die allgemeine Vorgehensweise unserer Arbeiten beinhaltet die Kombination von theoretischen und experimentellen Techniken. Wenn nötig, werden die entsprechenden Methoden im Hause entwickelt. Des Weiteren ist die Abteilung weltweit in zahreiche wissenschaftliche Projekte eingebunden.