Organometallic Chemistry
The research in the Department of Organometallic Chemistry is focused on the development of organometallic reagents and catalysts, investigations into their mode of action, as well as on applications to the synthesis of structurally complex targets of biological significance. Particular attention is paid to the development and validation of catalytic methods for C-C-bond formation. Long-term projects of current interest relate to alkene and alkyne metathesis, the development and use of pi-acid catalysts (platinum, gold etc.), metal carbene chemistry in general, as well as iron catalysis.
All methods are scrutinized by applications to the total synthesis and "diverted total synthesis" of natural products and pharmaceutically active compounds.
Research Topics:
Olefin metathesis is undoubtedly one of the biggest success stories of homogeneous catalysis during the last decades and, as such, was rapidly embraced by the synthetic community. The related metathesis of alkynes, in contrast, is much less commonly used, even though the reaction benefits from a surprisingly strong driving force and is mechanistically fairly well understood. The classical alkyne metathesis catalysts are rather difficult to make, highly sensitive and therefore of limited significance...
[more]
The keen interest in alkene metathesis dates back to the outset of Prof. Fürstner's independent career in the early 1990s. Our initial work showed the outstanding performance of the then brand new molybdenum and ruthenium alkylidene complexes developed by Schrock and Grubbs, respectively, for the preparation of medium-sized and macrocyclic rings. Rules were spelled out how to implement ring closing metathesis into retrosynthetic planning and examples were given, which demonstrated the complementary logic of this transformation.
[more]
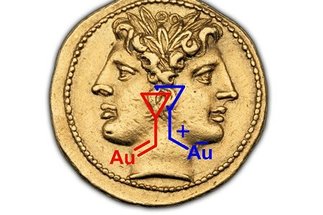
Catalytic activation of π-systems with the aid of carbophilic Lewis acids such as Pt(2+) and Au(1+) has gained considerable momentum only after the turn of the millennium. Except for a few pioneering studies, this field was virtually inexistent until the late 1990’s but is currently one of the most rapidly growing areas of homogeneous catalysis.
[more]
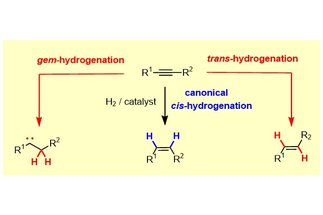
During the course of a catalytic hydrogenation, both H-atoms of H
2 are transferred to the same side of the p-system of a given substrate (“syn”/”suprafacial”). This paradigm remained basically unchallenged since the time when catalytic hydrogenation was discovered over a century ago. Our group managed to show that this stereochemical rule can easily be overcome with the aid of simple ruthenium catalysts, which allow internal alkynes to be reduced to E-alkenes with high levels of selectivity...
[more]
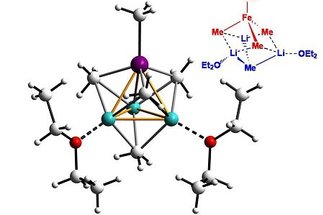
We are interested in the development of catalysts based on cheap, non-toxic, benign and readily available transition metals as substitutes for traditional noble metal complexes. Although not limited to, iron catalysis is currently the focal point...
[more]
Various lines of research are pursued by which we intend to establish new principles for catalysis and to develop conceptually novel ligand architectures....
[more]
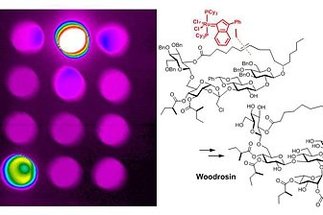
We are dedicated to the application of organometallic catalysis to the total synthesis of structurally complex natural products of biological significance; where indicated, we are also committed to prepare analogues by “diverted total synthesis” for biological and/or pharmacological evaluation.
[more]
Award Ceremony took place at Nagoya University
more
Organic Chemistry Award Ceremony took place during a EuChemS conference in Dublin.
more
Prof. Alois Fürstner visited the Netherlands and Spain
more
Alois Fürstner honored with the Heilbronner-Hückel Lecture
more
Meet Saskia Schulthoff, technician and chairperson of our works coucil.
more
Show more
Research Reports: